The Ultimate Guide of Gold Cyanidation
2018-11-2114:02:22XinHai Views:11496
The cyanidation gold extraction process is the main method for extracting gold from ores or concentrates, which is mainly divided into two processes, namely, tank leaching cyanidation method and heap leaching cyanidation method. The heap leaching method is mainly used to treat low-grade gold ore. The tank leaching cyanidation method is a traditional immersion gold method, mainly divided into two types: percolation cyanidation process and agitation cyanidation process. Compared with heap leaching, the tank leaching process is more widely used and is suitable for leaching gold ore with more complex properties.This guide will walk you through more main issues about gold cyanidation, you can use the table of contents on the right to navigate through the guide.
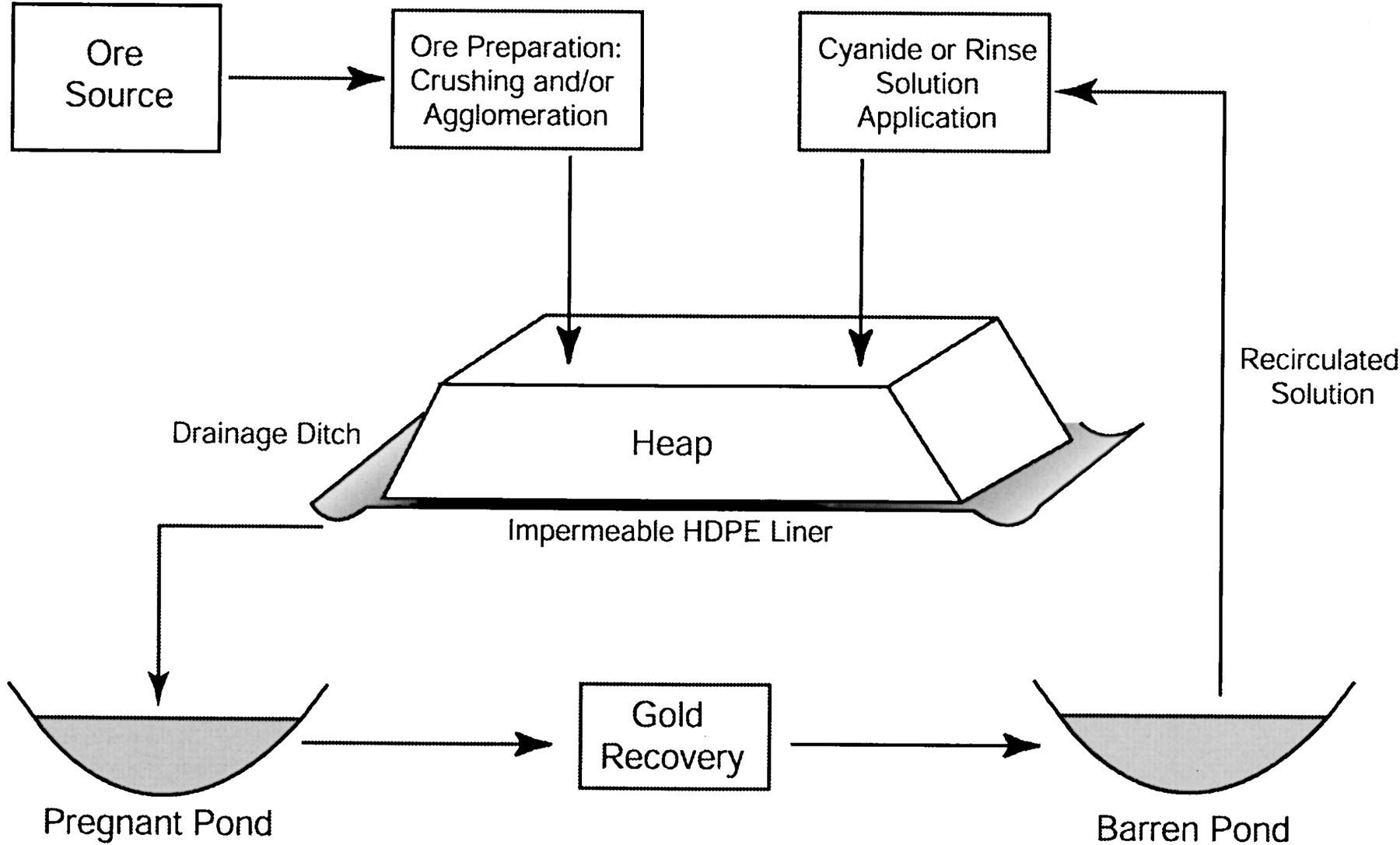
Heap leaching is a method in which a leaching solution is sprayed with a spray leaching system to selectively leach the useful minerals in the ore and recover the useful mineral from the pregnant liquid flowing out of the heap during the infiltration process. Low-grade gold ore heap leaching is a new gold extraction process developed in the 1980s. It is characterized by simple process, easy operation, short process, small floor space, strong adaptability, large or small scale, and low investment. The production cost is low. The disadvantage of this method is that the leaching rate is lower; generally only 60-80% can be recovered.
The Simple Operation of Gold mine heap leaching is following:
The industrial cyanide heap leaching method can be divided into two types, one is short-term heap leaching and the other is long-term heap leaching.
In the short-term heap leaching method, the ore is usually crushed to less than 20 mm. When processing gold-bearing quartz veins, it is often crushed to less than 6 mm. The crushed ore heap is leached on a permanent bottom mat, with stack height of generally 3-6 meters, per heap processing volume of 100-10000 tons, and per heap leaching cycle of generally 7-30 days. After a cycle of leaching is completed, the waste is removed from the bottom mat, and then a new batch of crushed gravel is piled up for the next cycle of leaching.
During long-term heap leaching method, using open-pit mining of loose and unbroken low-grade ore, which may contain some giant gravel, but most of the feedstock is less than 150 mm, and the capacity is 10,000-2,000,000 tons of ore per heap. The heap is similar to a truncated cone with 6-10-meter height. This heap has a long leaching period of about 4-5 months, which is based on the principle of leaching most of the useful mineral in the heap and may require a leaching period for several years. After the leaching is completed, the waste is piled on the bottom pad.
Conditions that must be met for ore that is suitable for heap leaching:
Heap leaching is often used to develop small-sized gold deposits or low-grade ore (1-3 g/t), or those small low-grade gold mines that cannot be developed and utilized by conventional methods.
The heap-leaching field has little requirement in terrain conditions, and can be adapted to local conditions. According to the topographical features, permanent unloading yards or superimposed yards are respectively set up. For example, the top of the mountain and the hillside are slower and wider, thus suitable for a permanent unloading yard.
The heap leaching process to extract gold is as following:
This includes crushing, screening or granulation and pretreatment. In order to improve the leachability of the ore and the permeability of the heap, avoid the phenomenon of uneven flow, blockage, channeling, etc., improve the metal recovery rate, reduce the consumption of the solution and improve the leaching effect, the ore must be broken to be suitable for leaching. Those ores containing more clay or mineral powder need to finely crushed to 6mm or less for granulation, that is, after adding a certain amount of water (30-40kg/t) and immersion liquid (25kg/t) in the crushed ore, stirring them in the granulator to bond the clay and the ore powder to the coarse particles and form a granular mineral, which can be used for pile-up after drying. If the content of clay and mineral powder of -0.075mm in the mineral material is high (8% to 10%), it is necessary to add a binder. The commonly used binders are cement (2~4kg/t) or lime (1.5~5kg/t). Sometimes in the granulation process, the leaching solution is added to pretreat the ore. The purpose of granulation and pretreatment is mainly to improve the permeability of the leaching dump and the leaching rate of the useful mineral.
In order to collect the pregnant solution and prevent the solution from leaking, the heap yard needs to be trimmed and padded (or bottomed) before ore stacking. The materials for the leaching mat construction are clay, sand, gravel, tailings, asphalt, reinforced concrete, plastic film or rubber plastic board, etc. Depending on the material, the thickness of the mat is generally 300-600 mm. The longitudinal and lateral drainage grooves are paved with lump ore on the mat, and a large ore is further laid on the entire bottom to protect the bottom mat.
The purpose of ore stacking is to make the heap have good and uniform permeability and to ensure that the side slope does not collapse. The height of the heap depends mainly on the permeability of the heap, the amount of the leaching agent in the leaching solution, and ore stacking equipment and methods. The height of the gold ore heap is generally 1 to 4 m, and the copper ore and uranium ore heap generally 3 to 30 m. The ore volume of the heap is generally from several hundred tons to several hundred thousand tons, and there are millions of tons of copper-poor heaps. The ore stacking method includes a multi-layer construction method, a multi-stack construction method, a slope pile-up method, and a moving bridge type pile-up method. The pile-up equipment mainly includes scrapers, front-end loaders, dump trucks, bulldozers, belt conveyors and special pilers.
The water and the leaching agent are formulated into a solution for leaching the useful element from the ore, which is also called a working solution. The formulation of the leaching solution and its amount are mainly determined by the mineral, mineral composition and chemical composition. In industrial-scale heap leaching, the available leaching agents are H2SO4, HNO3, HCl, Na2CO3, NaHCO3, (NH4)2CO3, NH4HCO3, Fe2(SO4)3, NaCl, KCl, Nal, Kl, (NH4). 2SO4, air, oxygen, permanganate, nitrogen oxides, hydrogen peroxide and chlorate. In order to accelerate the leaching of sulfide minerals and certain useful element of the oxidized ore, a bacterial liquid is also added to the leaching solution.
Spray the immersion solution evenly on the top surface of the heap. The liquid distribution (spray) system is composed of a liquid mixing tank, a pump, an infusion tube, and a spray pipe and a shower device laid on the heap. Plastic pipes are commonly used as sprinkler pipes, and showers are often swing type. The first requirements for spraying are to spray the leaching solution evenly on the heap. The second is to achieve the required spray intensity, that is, the spray amount per unit area per unit time, usually 0.1 to 0.4L. /m·min. In the heap leaching process, the spraying time accounts for about 1/3 to 1/2 of the total leaching time; in addition to the spray method, there are also other methods such as irrigation and drip irrigation. The liquid collection method of heap leaching is relatively simple, in which the rich liquid from the bottom of the pile is transferred into the sump through the liquid sump, and then pumped to the workshop for processing.
The process technology for recovering metal from rich liquid depends on the mineral types: for extraction of uranium, the common methods are extraction and ion exchange precipitation method and solvent extraction - electrowinning method; while for extraction of gold, common method is activated carbon (or ion exchange resin) adsorption - electrowinning method and displacement precipitation method.
With the continuous development of gold production and the rapid development of gold resources, the process of agitation gold leaching is also improving day by day, and has been widely used.
The agitation leaching method is to concentrate the slurry, obtained after grinding and classifying of the gold-containing ore, to a suitable concentration, place it in a leaching tank, add a cyanide solution, and aerate to carry out leaching. The main equipment used in the agitation leaching process is a cyanide leaching tank. According to the different mixing methods, cyanide leaching tanks are divided into three types:
Agitation leaching is suitable for process materials having a finer particle size, i.e., less than 0.3 mm. The advantages of this method are: high leaching speed, large processing capacity, mechanized operation, and high gold extraction rate. This conventional cyanidation method is one of the more widely used methods in the gold cyanidation process. According to the different ores processed, it can be divided into: the whole mud cyanidation method for directly processing gold ore, and the concentrate cyanidation method for process gold concentrate.
The process of the agitation leaching process comprises the following steps:
For general non-sulfide gold-bearing ores, it is usually grind to 60-70%-200 mesh. Cyanide is added to the grinding operation, and side immersion is performed to improve the leaching efficiency.
For sulfide gold-bearing ores, flotation enrichment is often used. Or separating out some gold-bearing sulfide concentrates from flotation concentrates, such as gold-bearing copper concentrates, and finely grinding them to 90%-95%, -325 mesh to shorten the leaching time and improve the leaching efficiency. For ore with higher arsenic or pyrrhotite, flotation concentrate ore roasting desulfurization or arsenic removal is carried out, and then the calcine is cyanated and leached. Ore with higher carbonaceous minerals, chlorinated and then leached.
The pulp is generally leached at a concentration of 35% to 50%, and the pH is adjusted to be between 10 and 10.5 to prevent decomposition of cyanide and inhibit the cyanidation of the sulfide. The concentration of cyanide is usually maintained at about 0.06%, and the mixture is agitated under oxygenation conditions for 24 hours or more, so that 95% or more of gold is dissolved to form a gold cyanide complex.
In order to obtain sufficient separation between the cyanide leachate and the leach residue, a washing process of 3-5 stages of thickening, filtration or a mixture of the two is generally employed. This is a key operation for cyanide leaching, which often results in a decrease in recovery rate due to the difficulty in selecting or incompletely separating the mud.
Maintain a high replacement rate and high grade gold mud, mainly using a clarification filter to reduce the suspended matter in the leachate from 70-100ppm to 5-7ppm, and deoxidize by vacuum to reduce the oxygen content in the solution to less than 1ppm.
The gold cyanide complex in the leaching solution is replaced with a metal to form a cyano complex of the replacement metal to precipitate gold. In order to obtain a more effective displacement reaction, a lead salt of about 0.005% is maintained in the solution, and the oxygen content in the solution is minimized to prevent oxidation of the zinc surface and reverse oxidation of the displaced gold, and precipitate the gold mud. Residual metal in the middle. It is removed by pickling to maintain a high-grade gold mud with a gold content above 30%.
The gold mud and the flux are generally smelted and slag at a furnace temperature of 1000-1100 degrees Celsius for about three hours at a ratio of 1:0.8-1 to obtain a gold ingot having a gold content of 95% or more.
The zinc powder replacement method is the most used metal replacement method. Zinc powder is a very fine metal substance that provides favorable conditions for more complete and faster precipitation of gold. However, zinc powder is easily oxidized and must be tightly closed during transportation or storage.
According to the different replacement methods in the separation stage, the agitation leaching method can be divided into two types of processes: metal replacement and carbon adsorption: The metal replacement is a so-called conventional cyanidation gold extraction process (CCD method and CCF method) which performs continuous countercurrent washing with a thickener and replaces the precipitated gold with zinc powder. Carbon adsorption is a non-filtered cyanide carbon slurry process (CIP method and CIL method) that does not require filtration or washing, and uses activated carbon to directly adsorb gold from cyanide pulp.
Since the 1980s, the agitation cyanidation method has achieved good results in the gold extraction process in the actual production process of gold.
Among the gold-bearing with pyrite ore and quartz ores processed by a gold dressing plant in China, the metal minerals mainly include pyrite, magnetite, pleatite, molybdenite, natural gold, etc.;The gangue minerals are mainly quartz, plagioclase, sericite and the like. The ore contains gold 6 g / ton, the gold particles are small, and gold and pyrite are closely symbiotic. The plant adopts a combined process of flotation and cyanidation. The gold sulfide concentrate is obtained after flotation. The grades are: gold 111.5 g/t, silver 43.17 g/t, copper 0.18%, aluminum 0.05%, sulfur 25.26%, carbon 1.54%. The recovery rate of gold flotation is 96%.
The gold-containing sulfur concentrate is leached by a agitation leaching system. The gold-containing sulfur concentrate is concentrated and decontaminated by a thickener and a filter, and is subjected to two-stage grinding to 98% -0.044 mm, and then carried out continuous cyanidation leaching in four mechanical agitation leaching tanks (φ3500 X3500 mm) with an inflator. The concentration of cyanide pulp is 25~30%, the pulp pH is 10, and lime is used as a protective base and add it to the section I mill. The sodium oxide concentration is 0.10%, the leaching time is about 36 hours, and the gold leaching rate is 94-86%.
The cyanide slurry after leaching is continuously washed with a double layer concentrator and a filter, and the total gold washing rate is 98% or more. The gold-containing solution (containing 20 g/m3 of gold) was clarified by adding a lead acetate solution, and then the gold was precipitated by a zinc wire replacement precipitation method. The replacement precipitation time was 90 minutes, and the gold substitution precipitation rate was over 99.
The gold mud is smelted into the ingot to obtain the gold and silver alloy, and the gold removal solution (containing gold 0.06 g/ton) is detoxified with bleaching powder or chlorine gas. The washed filter cake, cyanide tailings, is sent to the molybdenum flotation operation (adding No. 2 oil 60 g/ton, coal sleeve 80 g/ton. Water glass 8000 g/ton), where molybdenum is separated (semi-finished product, containing 13% molybdenum). The lead ore is oxidized and roasted, and the slag is processed by hydrometallurgy to obtain aluminate and tailings. The tailings are about 300 g/ton of gold, which is due to the adsorption of some strontium gold by the carbonaceous material in the gold-bearing concentrate. And caused by the loss of cyanide tailings. The tailings are smelted together with the gold-containing sulfur concentrate to recover the gold.
The molybdenum flotation tailings, that is, the cyanide final tailings, contain about 25 to 30% of sulfur and are sold to the sulfuric acid plant. The plant consumes material per kiloton of sulfur concentrate (kg/ton) as follows, NnCN8, lime 12, lead acetate 3, zinc wire 2-3.
Xinhai Mine designed a 1200 ton/day gold CIL dressing plant for a mining company in Tanzania. After the civil engineering, installation and commissioning of the process design, it has been put into operation. Finally, the CIL gold leaching rate is 91.5%, which brings considerable economic benefits to the mine owner.

After the crushing and grinding stage of the CIL gold ore process, the slurry is added to 9 Xinhai high-efficiency cyanide leaching tanks arranged in a stepped manner. The cyanidation of the gold slurry is first carried out in the first 2 leaching tanks, and adding activated carbon to the countercurrent adsorption operation while performing cyanidation in the subsequent 6-7 leaching tanks.
Compared with the CIP gold mining process and other traditional processes, the CIL gold mining process of the plant greatly shortens the cyanide operation time. Taking the 7.5g/t gold ore cyanide plant with a capacity of 100,000 tons per month as an example, the CIL carbon leaching method saves investment costs of 486,000 US dollars compared with the CIP carbon slurry method, reducing the backlog of gold retention in production of 210,700 US dollars. After deducting the cost of the $12.60 million spent on activated carbon, the savings and early recovery of funds were $675,100.
In the process of adding activated carbon, the imported coconut shell activated carbon (small hole, high activity, wear resistance and regenerability) specially selected by the plant's ore dressing design institute is added to the pulp, and the gold-loaded carbon appears with the gold and silver ions are dissolved and adsorbed by the characteristics of adsorbing gold and silver. This process does not affect the dissolution of the process due to excessive ion concentration, greatly improving the recovery rate of precious metals such as silver and copper in the associated metals, and significantly improving economic benefits.
The vibration and dewatering equipment is the key equipment for the reverse movement of pulp and carbon. The hydraulic filter press and high-efficiency high-frequency dewatering screen designed by the plant can effectively reduce the carbon wear of the slurry on the continuous pumping and vibrating screen surface and reduce the cost. Easy to maintain operations.
The central aeration riser used in the mixing process can make the slurry a small cycle. Compared with other mechanical agitation tanks, Xinhai's design can reduce the power consumption by 70%, and the solid materials are uniformly suspended, and the activated carbon wears little. The recovery rate is high. It is an important equipment in modern cyanide plants.
The integrated operation desorption electrolysis system of the plant uses high-temperature desorption electrolysis of gold in gold-loaded carbon using a mixture of sodium cyanide and sodium hydroxide. First, the wood scraps and other debris are removed by a pan wash, and then the gold desorption is performed at a temperature of 150 ° C and 0.5 Mpa using a high temperature and high pressure desorption method. This design can put 99% gold in 2-6 hours desorption down.
The concentration of gold and silver cyanide complex ions in the pregnant solution obtained by desorption of gold-loaded carbon is higher, and the impurity ions are greatly reduced. It provides an ideal solution for the reduction and recovery of gold from the noble liquid by electrowinning method. The high-purity solid gold can be obtained safely and economically by the treatment of the desorption electrolysis system.
The percolation cyanidation process is suitable for the treatment of alluvial sand, loose and porous materials, and slag. The percolation cyanidation process can handle materials up to -10 mm. If clay, slime or finely ground materials contained in the ore, the percolation effects will decline. Therefore, the slime must be separated before percolation cyanidation method is used. Sludge and finely ground materials are usually treated by agitation cyanidation.
Percolation cyanidation method is a relatively simple and inexpensive method of extracting gold. The advantage of this method is that it consumes less solvent and less power, and eliminates the need for expensive concentrators or filters. Compared with the agitation cyanidation method, the disadvantages of the percolation cyanidation method are: long working time, large equipment, large floor space, incomplete washing and low extraction rate of gold, thus often only suitable for small-scale gold mines, especially individual gold mining.
The ore ready for leaching is first filled in a percolation leaching tank (pool) and then leached with a cyanide solution. The cyanide solution slowly permeates the ore layer to dissolve the gold. The gold-containing solution (pregnant liquid) passes through a filter bottom (false bottom) slightly higher than the bottom of the tank, and flows out of the pipe on the wall of the tank. The tube is located between the bottom of the tank and the bottom of the filter. After the alluvial ore is treated with the cyanide solution, it is washed with water to wash out the gold-containing solution remaining in the ore.
The gold-containing solution flowing out of the tank is sent to a displacement precipitating device to precipitate gold. The precipitated de-golden poor liquid is sent to the lean liquid pool, and the appropriate amount of cyanide is added to increase the concentration for the next batch of new ore. The ore after washing with water is the tailings of percolation cyanidation and are discharged manually or mechanically, and then the next batch goes on operation.
The traditional cyanidation gold extraction process, namely the CCD method, is mainly composed of three processes of leaching, washing and displacement (precipitation).
The first process, leaching. After the preparatory stage prior to the completion of the separating, the gold in the ore is dissolved with an oxygenated cyanide solution. The two main agents used in the leaching process are cyanides such as sodium cyanide, potassium cyanide, and protection of alkali such as lime.
The second process, washing. The ore is subjected to cyanidation leaching to produce a slurry composed of a gold-containing solution and a tailings for washing and filtering, thereby separating the gold-containing solution from the solid tailings. The commonly used separation process is: concentration and filtration of cyanide pulp, and then washing the filter residue on the filter with de-gold depleted liquid or water, and then the solid containing lower gold, ie tailings, is discarded or reprocessed, and the gold-containing solution is used. Replacement precipitation with gold. In the solid-liquid separation, the washing water is added, and the washing water is generally used as a lean liquid or clean water discharged from the displacement operation.
In order to obtain sufficient separation between the cyanide leachate and the leach residue, a 3-5 stage dense-filtration or two mixed washing process is generally employed. When the processed ore contains less harmful cyanide impurities, all the lean liquid can be returned to the leaching operation. At this time, clean water is generally used as the washing water, which can improve the washing efficiency and make the cyanide tailings. The concentration of sodium cyanide in the solution is reduced, the loss of sodium cyanide is reduced, and the sewage treatment operation is simplified.
At present, there are many methods for separating the gold-containing solution and the tailings from the slurry, and the commonly used methods include a decantation washing method, a filtration washing method, and a fluidized washing method. In the production practice, which washing method and equipment are chosen is the key to improving the washing efficiency and reducing the production cost.
The third process, zinc powder replacement. The principle of zinc replacement gold is to add metal zinc to the pregnant solution after purification and deoxidation. After the displacement reaction, the gold in the solution is replaced by a metal state and precipitated, and the zinc is dissolved in the alkaline cyanide solution. The reaction is rapid and the replacement is complete. Its reaction formula is as follows:
2Au(CN)2-+Zn=====2Au↓+Zn(CN)42-
The residual metal in the precipitated gold mud is removed by pickling to ensure high-grade gold mud with a gold content of 30% or more. In the process of smelting the ingot, the solution of the finally obtained gold mud is smelted and slag at a furnace temperature of 1000-1100 ° C for about 3 hours according to a certain ratio, and a gold ingot having a gold content of 95% or more is obtained. The solvent ratio is 30-40% of borax, 25% of nitrate, 15-20% of quartz sand, 5-10% of fluorite, and soda, manganese oxide and son on.
The gold all-slime cyanidation CIP method or gold all-slime cyanidation CIL method is a new process for processing precious metal-containing pulp on the basis of the conventional cyanide gold extraction method. The three key steps in CIP and CIL process are:
There are some differences between the CIP and CIL processes. In the CIP process, leaching and adsorption are two operations, the leaching tank is used for cyanide leaching of the slurry, and the carbon slurry tank is used for adsorption of gold by activated carbon. In the CIL process, the leaching and adsorption of the slurry is an operation. These two steps are carried out in the same tank, so it is generally referred to as a leaching tank or a carbon slurry tank.
The CIP process is mainly consist of the preparation of leaching raw materials, agitation leaching and countercurrent carbon adsorption, desorption of gold-loaded carbon, electrolysis, smelting ingots, and auxiliary operations for activated carbon regeneration. It is characterized by two operations, namely leaching adsorption and desorption of gold-loaded carbon, instead of leaching, washing, and clarification. Since the gold-loaded carbon after the adsorption of gold is separated from the leaching residue (cyanide slurry), it can be carried out on a simple screening equipment, and can be washed and easily separated. It eliminates the interference of argillaceous minerals and thus has a wider adaptability to various minerals.
The leaching operation in the CIP process has the same leaching conditions as the conventional cyanidation method, and is generally leached by a continuous cyanidation method in the leaching tank, and the cyanide required for the leaching process can be added in the agitation tank. It can be added during the grinding process, and the gold in the slurry is leached by cyanidation with activated carbon, and it is carried out in a agitation tank by air agitation or mechanical agitation. The adsorption agitation tank is often used in series. The slurry acts from the upper section to the next section make use of its gravity, and finally is discharged by the pump. The activated carbon is loaded into each adsorption agitation tank, and the adsorption agitation tank is equipped with an interstage filter, the air lifter and so on.
In the carbon adsorption process, screens are used in many places, the purpose is to first screen out the wood and coarse ore in the slurry, often using a spacer screen or a vibrating screen.
Finally, it is the desorption of gold-loaded carbon, this operation is carried out by dissolving the gold attached to the surface of the activated carbon in a solution, and then reusing the carbon after activation. The gold-containing noble liquid after desorption can have a gold content of up to 600 g/m3. After filtering, the pregnant solution enters the electrolytic cell for electrolysis. The gold is in the form of flakes or flocs, which are deposited on the anodized titanium plate, and then scraped down to concentrate the ingot.
Since the selective adsorption of gold cyanide complex by activated carbon is stronger than the adsorption of silver cyanide complex, when the amount of both metals is high, the silver cyanide complex is displaced without being adsorbed. Or maybe the silver cyanide complex is adsorbed but then exchanged with gold cyanide complex; the higher the mass fraction of gold in the solution, the stronger the exchange substitution effect, resulting in a large loss of silver. The method to improve the recovery rate of dissolved silver is as follows:
1. Accelerate the turnover rate of activated carbon and control the operation at a lower gold loading amount to reduce the exchange of silver on the gold-loaded charcoal.
2. Segmental adsorption, that is, the leaching of the slurry into the adsorption section first adsorbs gold under the high gold mass fraction, and then adsorbs the silver under the low gold mass fraction. According to the gold and silver content, there are two different adsorption systems to obtain gold-loaded charcoal and silver-loaded charcoal respectively.
Either way, the operation volume of desorption and regeneration is increased. Therefore, it is necessary to determine the adsorption work system after technical and economic comparison according to the specific conditions to ensure high recovery rates of both gold and silver.

The key to improving the recovery rate of percolation cyanidation is to enhance the permeability of the heap and make the leachate fully contact and react with the free gold in the ore. In addition, increasing the oxygen content during the gold leaching process is also an important condition for increasing the leaching rate. In order to increase the recovery rate of gold when using percolation cyanidation method, the following measures can usually be taken:
The core problem of the percolation cyanidation is how to ensure that the gold extraction solution and the valuable components in the ore can be fully contacted and effectively reacted. This requirement is more difficult for ores containing more ore and clay.
During the granulation process, the powder ore in the clay and ore adheres to the coarse particles to form a fine-grained coating. The ore particles formed in this way have sufficient wet strength and are less likely to break when solidified and then wetted.
Granulation can produce porous and permeable raw materials, thereby overcoming the main problems caused by ore particle size segregation during the pile-up, such as migration of fine particles and channeling of the solution during leaching, thereby increasing the recovery rate.。
The basic principle of increasing the recovery rate of percolation cyanidation with a wetting agent is receiving great attention.
In theory, the addition of a special surfactant, a wetting agent, to the leachate reduces the surface tension and allows the ore and leachate to be more completely contacted, thereby increasing permeability and recovery rate.
Increasing the permeability can shorten the time required for leaching, reduce the cost of cyanide and pumping, reduce the dry zone in the leaching tank, enhance the penetration to individual ore particles, and finally achieve the purpose of improving the metal recovery rate.
A wetting agent is a material that enhances leaching. It not only increases the speed of leaching and final recovery rate, but also ensures that it does not adversely affect subsequent gold adsorption and desorption.
After the ore has been ground, it must be well classified, and then cyanidated separately according to the particle size to increase the percolation speed and recovery rate.
Prior to leaching, the ore may be washed with water, alkali or acid to reduce cyanide consumption and increase gold recovery.
For example, the free acid and soluble salts can be washed away with water; the acid can be neutralized with base; the copper oxides and carbonates can be eliminated with dilute sulfuric acid.
The above are several measures that can improve the recovery rate of the diafiltration cyanidation and accelerate the dissolution of gold to obtain higher beneficiation index.
Cyanide is an essential agent for gold extraction by cyanidation. Commonly used cyanide agents are NaCN, KCN, NH4CN, CaCN2 and cyanide melts. The cyanide melt is a cheap cyanide, the useful component is 45% CaCN2, and the rest are impurities such as soluble sulfide, carbon and insoluble matter. Before use, the sulfide is vulcanized by vigorously stirring the solution and adding lead salt to it. Lead precipitation, clarified solution for cyanidation.
When selecting cyanide, consider the relative solubility of cyanide to gold, stability, impact of impurities on the process, price and reliability of supply. The relative consumption of cyanide for equal solubility: KCN>NaCN>Ca(CN)2> NH4CN; cyanide can be decomposed into HCN in air containing carbon dioxide, its stability: KCN>NaCN> NH4CN> Ca(CN )2.
The most commonly used in industrial production is NaCN. It should be pointed out that according to the basic reaction formula of cyanide-dissolving gold, the theoretical dissolution of 1 gram of gold requires only 0.49 g of NaCN, but in actual production, the actual consumption is more than 200 times the theoretical consumption due to mechanical and chemical losses.

Sodium cyanide is a highly toxic agent, Germany, the Czech Republic, Hungary, Costa Rica, Montana and Wisconsin, and several Argentine provinces have imposed bans on the exploitation and use of sodium cyanide. Although other countries have not banned the exploitation and use of sodium cyanide, they have certain restrictions on their storage and transportation.
In 2002, Costa Rica suspended the opening of cyanide leaching mining.
Some provinces in Argentine prohibit cyanide mining, but there is no ban at the federal level.
Cyanide mining, open-pit mining and extraction of metals are prohibited in Chubute province. (August 5, 2003)
Rio Negro province bans the use of cyanide in the extraction, development and industrialization of metals (21 July 2005)
Cyanide mining, open pit mining and extraction of metals are banned in Tucuman province (20 April 2007)
The use of cyanide in metal detection, exploration, development and industrialization is banned in Mendoza (20 June 2007)
La Pampa province bans open pit mining, extraction of metals and the use of cyanide for exploration, development, extraction and storage of metals (16 August 2007)
Cordoba province bans open pit mining, extraction of metals and the use of cyanide for exploration, development, extraction and storage of metals (24 September 2008)
Rioha province bans the use of cyanide to extract metals (3 August 2007), but the ban was lifted (26 September 2008)
According to the Citizens 137 initiative approved by the US Montana Environmental Information Center on November 6, 1998, the state of Montana prohibits the use of cyanide for gold mining and open-air heap leaching. The Montana Supreme Court explained that the ban does not constitute a violation of the US Constitution.
According to the Colorado summit in the United States, Costilla, Gunnison, Conejos and Gilpin counties prohibit cyanide mining. However, the Colorado Supreme Court ruled in a Decree of the Colorado Mining Association Complaints Commission that a county that is a state branch may not ban chemicals allowed under the Colorado Mining Land Reclamation Act, and federal law(encourage exploration, mining and extraction of valuable minerals) takes precedence over county regulations.
The European parliament voted in 2010 to urge the European commission to enact a complete ban on cyanide extraction, but the commission has refused to recommend legislation. The main reason is likely to be that banning cyanide gold extraction in Europe would be bad for jobs, according to people familiar with the matter.
In 2002, the Czech parliament decided to ban gold cyanide leaching.
In 2006, Germany gradually reduced the amount of cyanide allowed in mining.
In December 2009, the Hungarian Parliament voted in a campaign organized by the Hungarian low cyanide Association to completely ban cyanide mining.
In 2007, the Turkish State Council decided not to allow cyanide mining, based on Article 56 of the Turkish Constitution: “Protecting people's right to live in a healthy environment.”
The Central American country El Salvador bans all forms of metal mining in the territory.
The El Salvador parliament voted on March 29, 2017, and a comprehensive ban on mining of metal mines was passed with the support of 70 members of different parties. This means that all metal exploration, refining and processing are prohibited, whether on the ground or underground; toxic chemicals such as cyanide and mercury are prohibited.
Sodium cyanide is classified as a Class 6 dangerous product in the Greater Vancouver Area of Transportation (the first level is the highest).
Regulations on the Safety Management of Chemical Dangerous Goods (promulgated by the State Council on February 17, 1987), Regulations for the Administration of the Safety of Chemical Dangerous Goods ([1992] no. 677), Regulations for the Safe Use of Chemicals in the Workplace ([1996] ministry of Labour no. 423) have made corresponding provisions on the safe use, production, storage, transportation, loading and unloading of sodium cyanide.
According to Zimbabwe's Hazardous Substances Act, cyanide can only be sold to qualified customers in the mining and chemical industries by qualified chemical distributors. Most of these customers are gold miners who need to write documentary evidence that they have the right to hold and use cyanide.
Qualified chemical distributors must obtain a license from the Mining and Mineral Development Department.

With the increasingly prominent contradictions between economic benefits and environmental protection, the requirements for the development of low-toxic and environmentally-friendly gold-dressing reagents as alternatives to cyanide for leaching gold ores are becoming more and more urgent. Research and development of low-toxic gold-dressing reagents with high performance and high market prospects plays an important role in the development of the gold industry. In order to solve the problem of highly toxic pollution of cyanide, for decades, scholars at home and abroad have carried out a large number of experimental research work, and strive to find a low cyanide, low-toxic or low-toxic beneficiation reagent as alternatives to cyanide. Finally, several leaching reagent had been researched, such as: thiourea, water chlorine, coal agglomeration, thiosulfate, stone sulfur, hypochlorite, etc., but for various reasons these alternatives to cyanide have not achieved industrial production and use.
Among them, thiosulfate is considered to be the most promising gold leaching reagent as alternatives to cyanide because of its rapidity and low-toxicity. However, when there is only thiosulfate in the immersion gold solution, the gold dissolution is very slow. In order to increase the leaching speed, the researcher adds copper (II) ions to the thiosulfate solution, and the ammonia water is used as a mixed solution as a gold extraction leaching solution. In the presence of copper ammine ions, although the gold dissolution rate is increased, the consumption of thiosulfate is sharply increased, and the products of the thiosulfate are numerous, which makes the gold leaching composition complicated, and the gold entering the leaching solution is difficult to be effectively recovered. With the defects of large consumption, the thiosulfate reagent is difficult to industrialize production and use.
With the call of the times, CNFREE eco-friendly gold ore dressing reagent that can be completely alternatives to the highly toxic sodium cyanide in gold leaching process appeared. 100% environmental protection. 100% safety.
Under other same leaching conditions, the economic benefit analysis of comparable items was carried out for the amount, leaching rate and gold adsorption rate. The comparison test results prove that CNFREE eco-friendly gold leaching reagent almost obtain the equal economic benefit for the gold mine as NaCN when used as leaching agents. From the perspective of environmental protection and safety, CNFREE is a perfect alternatives to cyanide for leaching gold ores.
Don't forget to share this post!

TOP